Healthcare
3 promising innovations for early diagnosis of lung cancer
In this article, our Healthcare team looked at the reasons for the difficulties of early diagnosis of lung cancer and especially at innovations to address these difficulties.
Recent years have witnessed significant progress in Antibody drug conjugates (ADC) development. Between 2019 and 2022, 8 ADCs gained FDA approval, and in total as of now, 12 ADCs received FDA approval. And many more are to come: there are more than 170 ADCs in the clinical stage of development, amongst which 57 just entered Phase 1 in 2022 (an increase of 90% compared to preceding year). Moreover, 249 clinical trials evaluating ADCs were initiated in 2022, marking a 35% increase compared to 2021.
The effectiveness of ADCs spans diverse tumor types, and their application is expected to expand as more suitable target antigens are identified and technological advancements occur. Global Data’s Intelligence Center reports that ADC sales approached $7.7 billion in 2022 and are projected to reach $38.7 billion by 2029, indicating a remarkable compound annual growth rate (CAGR) of 26%. These figures underscore the promising future of ADCs in cancer therapy, given their targeted nature and efficacy. In this article, Alcimed analyses how antibody drug conjugates are about to revolutionize cancer care.
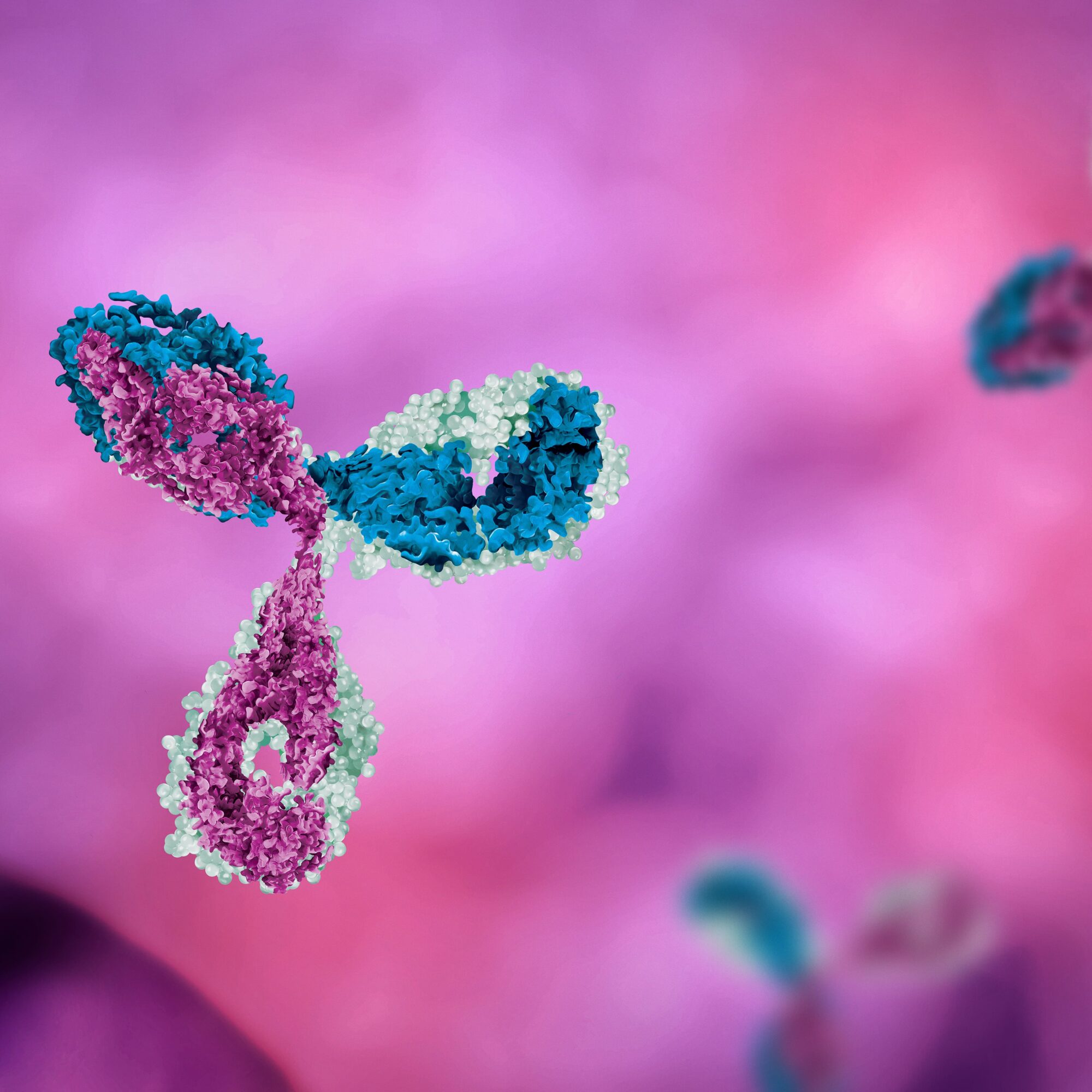
Antibody-drug conjugates (ADCs) are a targeted therapy used in cancer treatment, comprising an antibody, a cytotoxic drug, and a linker molecule. The antibody component specifically recognizes cancer cells by binding to their surface antigens, delivering the cytotoxic drug directly to the cancer cells while minimizing harm to healthy cells.. ADCs offer benefits such as improved outcomes, reduced side effects, resistance overcoming potential, and targeted drug delivery.
Antibody-drug conjugates (ADCs) embody an elegant mechanism that combines the power of antibodies and cytotoxic drugs to target and combat cancer cells. Through their three essential components, ADCs pave the way for a more precise and effective approach to cancer treatment.
First, ADCs utilize monoclonal antibodies specifically designed to recognize and bind to unique antigens present on the surface of cancer cells. This targeted recognition allows for the selective delivery of the therapeutic payload to cancer cells, sparing healthy cells from unnecessary damage. Each ADC is tailored to match the antigen profile of the specific cancer type being treated, such as the notable targeting of HER2 in HER2-positive breast cancer.
Once the ADC attaches to the cancer cell’s surface, the ADC is internalized into the cancer cell, propelled by receptor-mediated endocytosis. The complex formed by the antibody and antigen embarks on a journey to intracellular compartments like endosomes and lysosomes, laying the groundwork for the subsequent steps.
Within these intracellular compartments, a critical process takes place. The linker molecule, acting as the bridge between the antibody and the cytotoxic drug, undergoes enzymatic or chemical cleavage. This precise cleavage, triggered by factors specific to the intracellular environment, liberates the cytotoxic drug from its antibody carrier. The clever design of the linker ensures the drug’s controlled release within the cancer cell, safeguarding healthy tissues from unnecessary exposure.
Once released, the cytotoxic drug springs into action with remarkable potency. It disrupts crucial cellular processes, such as DNA replication or microtubule assembly, impeding the cancer cell’s ability to divide and grow. Even at low concentrations, the cytotoxic drug proves highly effective, driving cancer cell death and promoting therapeutic outcomes.
The intricate dance of the antibody, linker, and cytotoxic drug in ADCs represents a breakthrough in cancer therapy. By harnessing the targeting capabilities of antibodies, ADCs offer a tailored approach that delivers potent drugs directly to cancer cells while sparing healthy cells. This precise targeting seeks to maximize treatment efficacy while minimizing the side effects associated with traditional chemotherapy.
One challenge is the potential development of resistance. Cancer cells may acquire mechanisms to evade or eliminate ADCs, diminishing their effectiveness over time. Additionally, the heterogeneity of tumors, where different cancer cells express varying levels of antigens, can limit the efficacy of ADCs that target specific antigens.
Safety concerns also arise due to the cytotoxic nature of the drug component. Imperfect selectivity may result in off-target effects, where healthy cells are affected, leading to adverse reactions. Furthermore, the drug’s toxicity may impose limitations on the maximum dose that can be administered, potentially impacting the treatment’s efficacy on a local level.
To summarize, the field of antibody-drug conjugates (ADCs) is characterized by its dynamic nature. Despite initial setbacks and challenges, the number of approved ADCs and those undergoing clinical trials continues to rise steadily. This growth trajectory is expected to accelerate significantly in the coming decade. At Alcimed, we‘ll you updated on the latest developments in this exciting field. Should you have any project in this area, don’t hesitate to contact our team!
About the author,
Volker Bischoff, Great Explorer Oncology, in Alcimed’s Healthcare team in Germany